The big deal about ozone and the ozone layer is down to the fact that is has a direct connection to how much UV life receives here on the earth’s surface. The sun’s rays travel more than 90m miles to reach us on earth. These rays contain Ultra Violet Rays, commonly called UV rays. There are different wavelengths of UV, but they all are connected to the amount of ozone that is available to filter the good ozone from the bad ozone.
– Ozone is a natural gas composed of three atoms of oxygen.
– The chemical formulae of ozone is O3
– Ozone is blue in color
– Ozone has a strong smell.
– Ozone is known to be a very powerful oxidant, making it very damaging to mucus and respiratory tissue in plants and animals.
Good Ozone
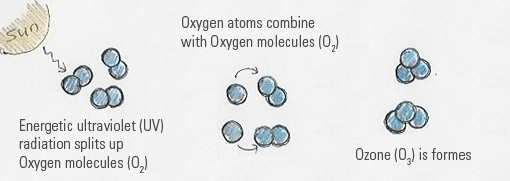
Good ozone (also called Stratospheric Ozone) occurs naturally in the upper Stratosphere. The stratosphere is the layer of space 6 to 30 miles above the earth’s surface. Where does good Ozone come from? The air is full of gases reacting with each other, even though our eyes do not see. When UV light strikes (Oxygen) O2 molecules, they are split into two individual O atoms — O and O. When one of the O atoms combine with O2 molecule, ozone (O3) is created. Even though Ozone is only a small part of the gases in this layer, it plays a vital role because it shields us from the sun’s harmful UV rays. It is called Good Ozone, for obvious reasons—because it protects humans, life and animals on earth.
Bad Ozone
Bad Ozone is also known as Tropospheric Ozone or ground-level ozone. This gas is found in the troposphere, the layer that forms the immediate atmosphere. Bad Ozone does not exist naturally. Human actions cause chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOC). Where does bad ozone come from? Each time there is a reaction of chemicals such as those found in cars, power plants and factory emissions, in the presence of sunlight (UV light), Bad Ozone is created.
